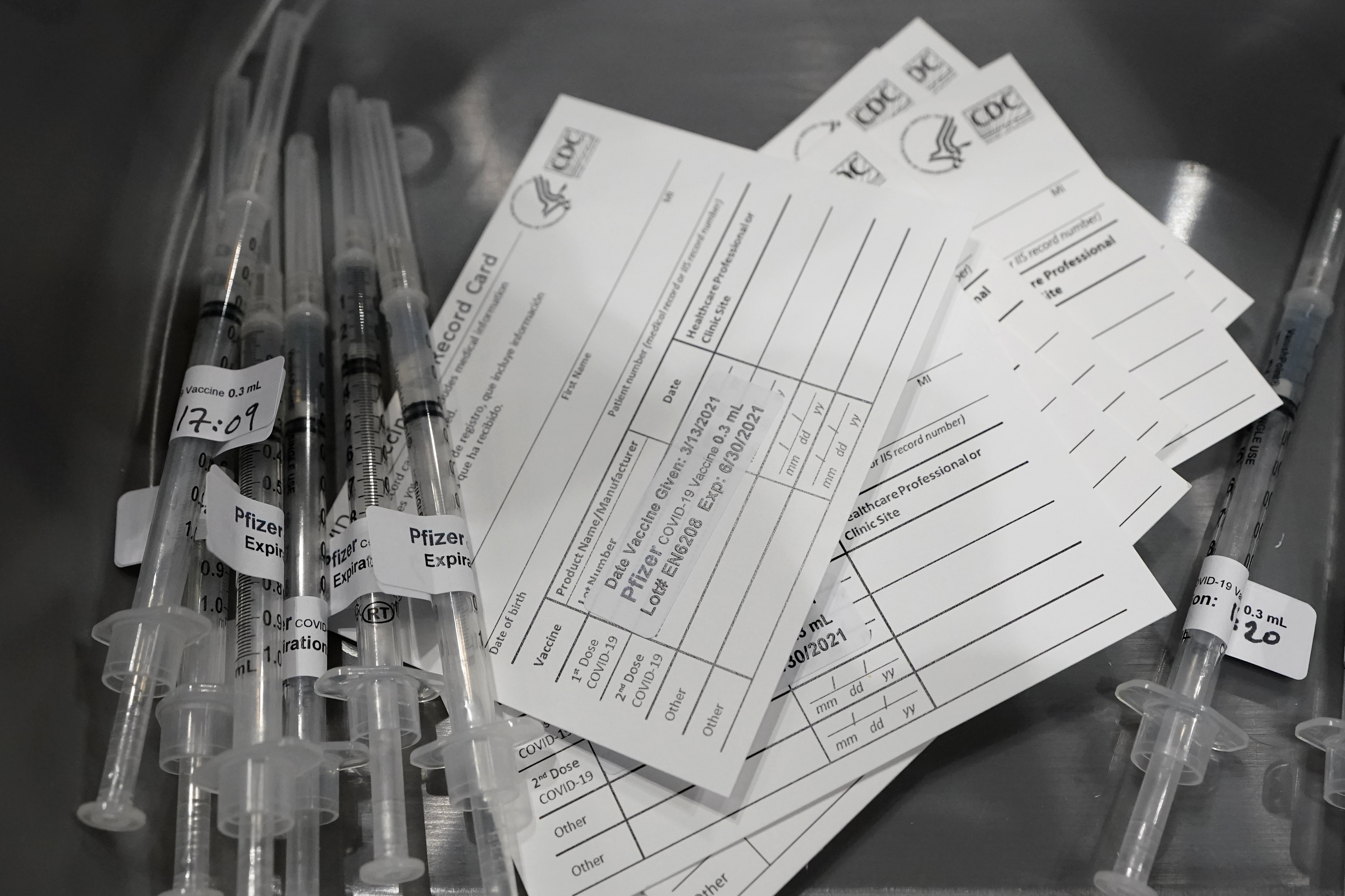
TAMPA, Fla. (WFLA) — Pending vaccine approval by the FDA, Florida expects to have two million doses of the coronavirus vaccine ready to go by the end of the year.
This week, a CDC panel recommended that states give those first doses to health care workers and long-term care facilities. Gov. Ron DeSantis has already said his priority is nursing homes.
Supply and logistical challenges will require releasing the vaccine in phases over several months, meaning that if you’re younger, relatively healthy, and don’t work in a health care environment, don’t expect access to the vaccine for several more months.
“They want to protect people most at risk first,” according to Dr. Michael Teng with USF Health.
The second roll out would likely be limited to essential workers and those over 65 with underlying health conditions. Some optimistic projections believe all Americans who want a vaccine will have one by June, but Teng is skeptical there will be enough to go around by then unless more than just the frontrunner vaccines from Pfizer and Moderna are approved.
There’s also the issue of those who opt-out. Studies have shown as many as half of Americans will not or are hesitant to receive the vaccine.
“This is a brand new vaccine,” Teng explained. “They have a little mistrust, they may not want to be vaccinated.”
Clinical trials found both the Pfizer and Moderna vaccines to be highly effective, but with side effects in some participants. The side effects described are very similar to those from the flu shot: fatigue, headaches, slight chills or fever.
In some participants, the side effects were more noticeable after taking the booster shot. The vaccines closest to approval all require a booster a few weeks after the initial vaccination to ensure the inoculation is effective.
Can you legally be required to take the vaccine? Yes, according to USF’s Dr. Jay Wolfson, who holds both medical and law degrees.
However, Gov. DeSantis has been adamant that he will not mandate the vaccine. One Florida lawmaker even filed a bill to try and prevent the state from doing so.
Wolfson thinks keeping the vaccine voluntary is wise because forcing it upon people could create even more distrust from those already leary and with vaccine access for the general population not expected until spring, he says there is still time to mobilize and convince Floridians getting the COVID-19 vaccine is the right thing to do.
“Voluntary participation is going to be very important,” he said.
Wolfson says roughly 70 percent of the general population will need to be vaccinated to achieve desired immunity results.
The FDA will review Pfizer’s vaccine on Dec. 10, followed by Moderna’s on Dec. 17. If and when emergency authorization is granted, the companies say they are ready to begin distribution within 24 hours.
LATEST ON THE CORONAVIRUS PANDEMIC:
- Omicron, storms, disrupt air travel for 4th consecutive day
- Loss of smell from COVID most likely means you’ve got a mild case, study finds
- Federal program offers cash to cover COVID-19 funeral costs
- COVID infections should no longer be ‘major metric’ of pandemic, health expert says
- Christmas COVID infections overwhelming Tampa testing sites